


random user submitted photo
Temperature Balancing by Turning/Twisting the Carb
42 posts
• Page 3 of 5 • 1, 2, 3, 4, 5
Re: Temperature Balancing by Turning/Twisting the Carb
Great work on the intake elbows. I thought the change would be greater. My left rear EGT is the hottest/leanest on climb out, but something I have noticed is that when that cylinder is getting hot, if I retard the throttle a small amount that cylinder will cool without a noticeable change in rpm. I thinking the throttle slide is not linear. At the wot position it goes lean and a small amount of change in slide position eliminates it. Just an unsupported observation.
From what I'm told by Bill Larson, the turbo is the best homogenizer for even temps. And, we all know that a warm/hot intake manifold runs better than a cold one. Lycoming design? Have you seen ice form on the manifold above the Aeroinjector? I'm thinking that more heat applied to the intake manifold wound increase atomization, reducing the heavy particles at the intake elbow. Ducting the top oil cooler over the pipes may be a simpler approach. Just saying.
From what I'm told by Bill Larson, the turbo is the best homogenizer for even temps. And, we all know that a warm/hot intake manifold runs better than a cold one. Lycoming design? Have you seen ice form on the manifold above the Aeroinjector? I'm thinking that more heat applied to the intake manifold wound increase atomization, reducing the heavy particles at the intake elbow. Ducting the top oil cooler over the pipes may be a simpler approach. Just saying.
OneX 107
N2107X
N2107X
- Onex107
- Posts: 506
- Joined: Mon Mar 24, 2014 6:44 pm
- Location: Peoria, IL
Re: Temperature Balancing by Turning/Twisting the Carb
If you go back a page you'll find the redesigned intake elbow I made to see if it would even out the mixture distribution on the AeroVee, with lackluster results. The conjecture was that a plenum with individual runners would take care of this. Well, here it is:
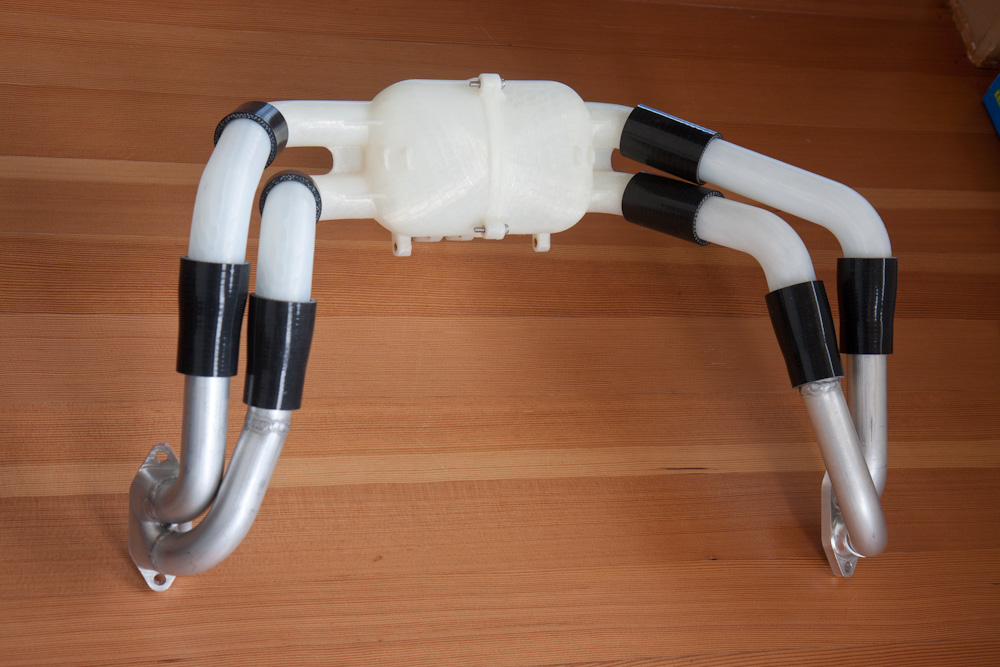
The plenum is 3d-printed in a high-temperature, fuel-resistant nylon alloy, with four equal-length runners going to the cylinders. (These runners are also plastic, they will be replaced with stainless but the plastic is fine for testing.)
On the engine:
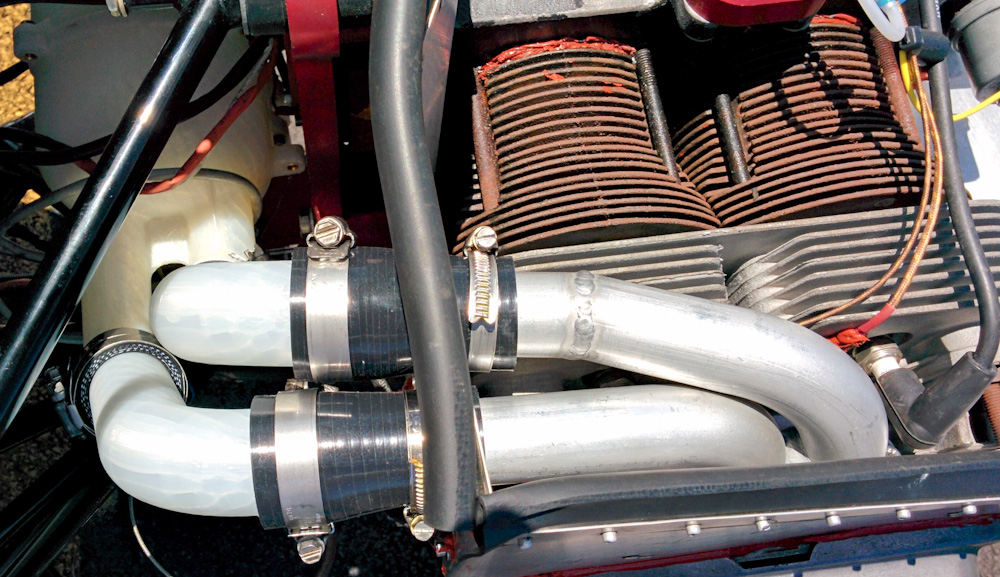
I finally did a test run today and even though it's not tuned yet, the EGT spread has come down to less than half of what it was before and as a bonus it also picked up 60 static rpm to 3220. It won't idle now, which is likely a vacuum leak somewhere, but I'm hopeful we're on the right track.

The plenum is 3d-printed in a high-temperature, fuel-resistant nylon alloy, with four equal-length runners going to the cylinders. (These runners are also plastic, they will be replaced with stainless but the plastic is fine for testing.)
On the engine:

I finally did a test run today and even though it's not tuned yet, the EGT spread has come down to less than half of what it was before and as a bonus it also picked up 60 static rpm to 3220. It won't idle now, which is likely a vacuum leak somewhere, but I'm hopeful we're on the right track.
- lutorm
- Posts: 259
- Joined: Mon May 15, 2017 1:35 pm
- Location: The Island of Hawai
Re: Temperature Balancing by Turning/Twisting the Carb
super cool - I love reading about this project.
-

kevinh - Posts: 372
- Joined: Mon Feb 23, 2015 10:46 pm
- Location: San Mateo, CA
Re: Temperature Balancing by Turning/Twisting the Carb
kevinh wrote:super cool - I love reading about this project.
Glad you like it. If you're interested, you can find all the gory detail on my blog.
- lutorm
- Posts: 259
- Joined: Mon May 15, 2017 1:35 pm
- Location: The Island of Hawai
Re: Temperature Balancing by Turning/Twisting the Carb
Excellent gory detail. I'm rooting for you too, it is a cool project with some real potential benefits.
Bryan Cotton
Poplar Grove, IL C77
Waiex 191 N191YX
Taildragger, Aerovee, acro ailerons
dual sticks with sport trainer controls
Prebuilt spars and machined angle kit
Year 2 flying and approaching 200 hours December 23
Poplar Grove, IL C77
Waiex 191 N191YX
Taildragger, Aerovee, acro ailerons
dual sticks with sport trainer controls
Prebuilt spars and machined angle kit
Year 2 flying and approaching 200 hours December 23
-

Bryan Cotton - Posts: 5489
- Joined: Mon Jul 01, 2013 9:54 pm
- Location: C77
Re: Temperature Balancing by Turning/Twisting the Carb
Turns out the 3D-printed plenum was very leaky. After coating it with epoxy the vacuum leak is gone and the engine runs and idles really well! Still have to re-tune the AeroCarb and I'm working on replacing the plastic intake runners with stainless, but this seems very promising.
https://blog.familjenjonsson.org/blog/2017/09/16/plenum-test-run-2/
https://blog.familjenjonsson.org/blog/2017/09/16/plenum-test-run-2/
- lutorm
- Posts: 259
- Joined: Mon May 15, 2017 1:35 pm
- Location: The Island of Hawai
Re: Temperature Balancing by Turning/Twisting the Carb
Must be at least 15 years ago I read about a theory that the fuel/air mixture coming out of the carb, in this case automotive, was not atomized sufficiently for ideal combustion. It's still a mist and not a vapor...vapor being the state that actually ignites which is why gasoline appears to be burning off the top of a gasoline puddle.
This fellow put some kind of very small ultrasonic black box in the intake manifold which resulted in better mpg, presumably because the transition between mist and vapor after passing over the ultrasonic gizmo was completed and now, only vapor was introduced into the combustion chamber.
I have no further knowledge of this experiment. Anybody else care to postulate a theory?
This fellow put some kind of very small ultrasonic black box in the intake manifold which resulted in better mpg, presumably because the transition between mist and vapor after passing over the ultrasonic gizmo was completed and now, only vapor was introduced into the combustion chamber.
I have no further knowledge of this experiment. Anybody else care to postulate a theory?
Darick Gundy
Sonex #1646
N417DG
Taildragger, Aerovee, center stick, Prince P-Tip Prop
MGL E1, F2, V6 radio, Sandia Xponder, Reserve lift indicator (AOA), iFly 520
First flight! 10/21/2017
Sonex #1646
N417DG
Taildragger, Aerovee, center stick, Prince P-Tip Prop
MGL E1, F2, V6 radio, Sandia Xponder, Reserve lift indicator (AOA), iFly 520
First flight! 10/21/2017
-

Darick - Posts: 496
- Joined: Mon Aug 05, 2013 9:39 pm
- Location: PA
Re: Temperature Balancing by Turning/Twisting the Carb
Darick wrote:Must be at least 15 years ago I read about a theory that the fuel/air mixture coming out of the carb, in this case automotive, was not atomized sufficiently for ideal combustion. It's still a mist and not a vapor...vapor being the state that actually ignites which is why gasoline appears to be burning off the top of a gasoline puddle.
This fellow put some kind of very small ultrasonic black box in the intake manifold which resulted in better mpg, presumably because the transition between mist and vapor after passing over the ultrasonic gizmo was completed and now, only vapor was introduced into the combustion chamber.
I have no further knowledge of this experiment. Anybody else care to postulate a theory?
It's certainly true the gasoline has to vaporize to burn. Maybe you can break up large drops with ultrasound, but in the end you have to supply the latent heat of vaporization. It would be interesting to know whether the ultrasonic gizmo also produced a noticeable lower temperature in the manifold.
I did a quick back of the envelope calculation for my situation. Fuel flow at full throttle is about 30l/h or 0.5l/min or 0.36kg/min. At 3000rpm the engine will pump 2.18*3000/2 = 3300 l/min air (assuming 100% volumetric efficiency) or 4kg/min. (Incidentally this is an air/fuel ratio of 11 which is in the right ballpark.)
According to some old military table I found, avgas has a latent heat of vaporization of ~375kJ/kg. To vaporize 0.36kg/min of avgas we thus have to supply 135kJ/min of heat. Air has a specific heat of 1kJ/kg*K so if all that heat were to come out of the air it would have to drop 135/(4*1) = 34 degrees C. Observed temperature drop is 11C, so by the time it's in the plenum, about 1/3 of the gasoline should have evaporated. The rest will presumably evaporate as it hits the hot intake tract and the intake valve.
It's interesting to note that the numbers come out to mean a significant fraction of the gasoline has evaporated already inside the plenum. And cooling the intake charge is always good -- the cooler the air, the more mass you can fit into the cylinders. (This is one of the reasons why you can get higher power out of an engine by running E85, ethanol takes a lot of energy to evapolate, so it will cool the intake charge much more than gasoline.)
- lutorm
- Posts: 259
- Joined: Mon May 15, 2017 1:35 pm
- Location: The Island of Hawai
Re: Temperature Balancing by Turning/Twisting the Carb
Your measurment is the first I've seen of temperatures after the Aerinjector. In my mind that measurement seems important to understanding the Aeroinjector's resistance to icing.
- gammaxy
- Posts: 600
- Joined: Wed Sep 04, 2013 9:31 am
Re: Temperature Balancing by Turning/Twisting the Carb
gammaxy wrote:Your measurment is the first I've seen of temperatures after the Aerinjector. In my mind that measurement seems important to understanding the Aeroinjector's resistance to icing.
Yeah, I think the key is that there's no venturi that lowers the pressure and temperature right where the throttle is, since it takes a while for the gasoline to evaporate. Even if the evaporation would take the air temperature below freezing, it doesn't have anything to freeze onto except the intake walls which are heated from the outside. This is unlike the throttle butterfly in a traditional carb which basically sits entirely inside the cooled airstream.
- lutorm
- Posts: 259
- Joined: Mon May 15, 2017 1:35 pm
- Location: The Island of Hawai
42 posts
• Page 3 of 5 • 1, 2, 3, 4, 5
Who is online
Users browsing this forum: No registered users and 48 guests







